Principles of Corrosion
2. PRINCIPLES OF CORROSION
2.1 HALF CELL REACTIONS
Corrosion is an electrochemical process in which metals and alloys undergo transformation into predominantly oxides, hydroxides, and aqueous salts.
In the corrosion process, two reactions take place. In one, the anodic reaction, metal atoms are ionised and pass into solution leaving their electrons within the original metal surface. In the second, the cathodic reaction, the free electrons within the metal are taken up by chemical species such as O2 and H2O in reduction reactions.
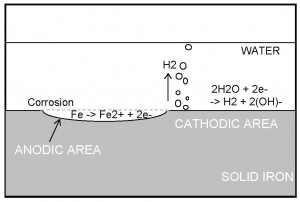
Consider a simplified version of the corrosion reaction between iron and water. The overall reaction proceeds as follows:
Fe + 2H2O → Fe (OH)2 + H2 (1)
The overall reaction can be broken down into the oxidising ANODIC reaction
Fe → Fe2+ + 2e- (2)
and the reducing CATHODIC reaction
2H2O + 2e- → H2 + 2(OH)- (3)
Figure 2.1 depicts this corrosion process.
The reaction 2 and 3 are called ‘half cell’ reactions. Reaction 2 is the half of the process which is responsible for the damage during corrosion. The speed at which this reaction proceeds is directly related to the corrosion rate.
2.2 ELECTRODE POTENTIALS
Values of ELECTRODE POTENTIAL are associated with each of the half cell reactions and give a measure of the likelihood for the reaction to occur. Figure 2.2 depicts standard electrode potentials as measured on the Standard Hydrogen Electrode Scale for some selected half cell reactions. This scale sets as datum a value of zero volts for the reduction of Hydrogen.
The more reactive the metal the more negative is its standard potential. In the case of Iron, it is -0.440V, whereas, for the more inert engineering metals, such as Platinum, the standard electrode potential is +1.20V.
This information allows us to determine whether a metal will corrode in a given environment.
FIGURE 2.1 – Schematic of the Corrosion Process
Half Cell Standard Electrode
Reactions Potential (Volts)
Zn → Zn2+ + 2e- -0.76
Fe → Fe2+ + 2e- -0.44
2H+ + 2e- → H2 0.00
2H2O + 2e- → H2 + 2(OH)- 0.40
O2 + 4H+ + 4e- → 2H2O 1.23
Cl2 + 2e- → 2Cl- 1.36
FIGURE 2.2 – Selected Standard Half-Cell Potentials
Under standard conditions, the potential of the corrosion cell is the difference between the cathodic reaction half cell potential and the anodic half cell potential. For reaction 1 the potential difference between the cathodic and anodic half cell reactions is:
E = +0.401 – (-0.440) = +0.841V
The positive value of the potential indicates that the reaction is possible and that corrosion will occur under these conditions. However, theory does not permit the calculation of the corrosion rate; this has to be measured, preferably in-situ. Thus a calculation of the corrosion cell potential is not able to predict the magnitude of damage likely to occur by the corrosion reaction.
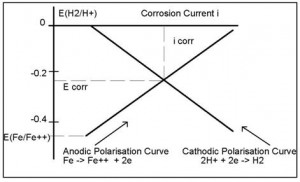
2.3 CORROSION RATES AND POLARISATION
The existence of a potential difference between the anodic and cathodic half cell regions generates a current flow. This has the effect of reducing the potential difference between the half cells, phenomena known as polarisation. The shape of the polarisation curve is affected by many factors, but in its simplest form is depicted in Figure 2.3.
At the point of intersection of the two polarisation curves, a metal will be freely corroding. The potential at which this occurs is called the Free Corrosion Potential (Ecorr) which generates a Corrosion Current (Icorr). From the corrosion current, it is possible to calculate corrosion rates.